We explore the discrepancy in the folding cooperativity of proteins in simulation versus experiment. The following figure shows dashed lines representing a more cooperative transition for heat capacity as a function of temperature and for fraction folded as a function of temperature. Solid lines represent a less cooperative transition.
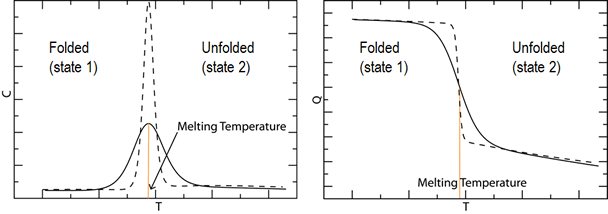
Simulation often gives results such as the solid lines, with broad transitions, while experiment often gives results such as the dashed lines, with narrow transitions.
Using a coarse-grain model, we have focused on two proteins, phage 434 repressor (1R69) and T4 lysozyme (7LZM).

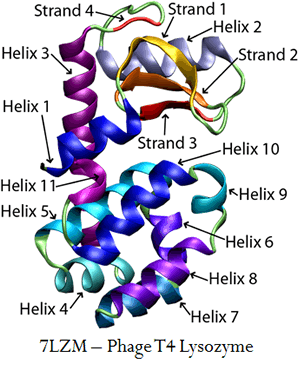
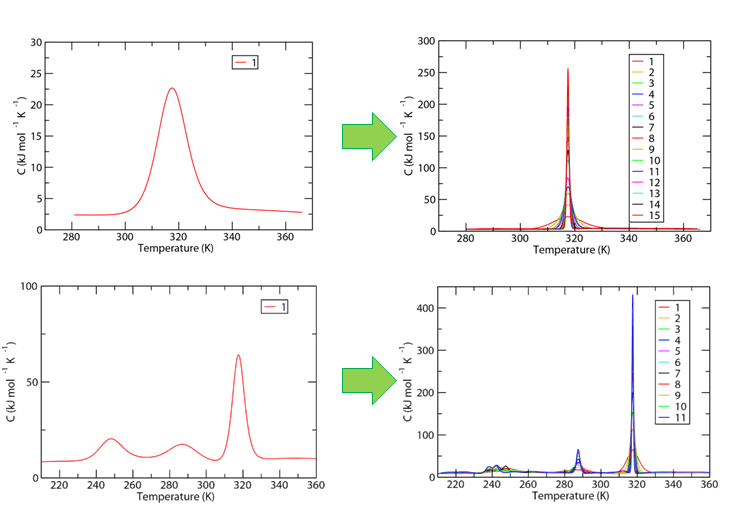
With these two proteins, we have looked into the effects on cooperativity when multiple proteins of one kind are simulated simultaneously using replica exchange. We hypothesized that approaching experimental conditions, where large magnitudes of proteins are examined, would help us approach experimental results. What we found is very interesting.
For 1R69 (the top row on the figure below), the single transition exhibited by 1R69 became increasingly cooperative as more and more proteins were simulated simultaneously. For 7LZM (the bottom row on the figure below), only certain transitions increased in cooperativity. All heat capacities shown are normalized to a per-protein basis.
Based on the equilibrium states of 7LZM between transitions, we infer that the degree of cooperativity of each transition affects how that transition changes as the number of proteins increases. For example, a less cooperative transition becomes increasingly uncooperative while the most cooperative transition shows the greatest increase in cooperativity. The degree of cooperativity, then, is compounded by having multiple molecules simulated simultaneously.
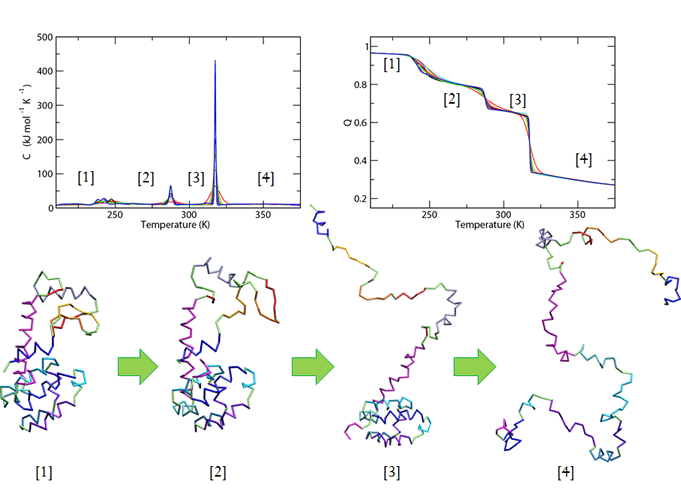
The transition between states 1 and 2 appears least cooperative. The transition between states 2 and 3 appears fairly cooperative, and the transition between states 3 and 4 appears most cooperative. We believe that the compounding due to the increased number of proteins makes the transitions reflect the inherent cooperativity of each transition better.